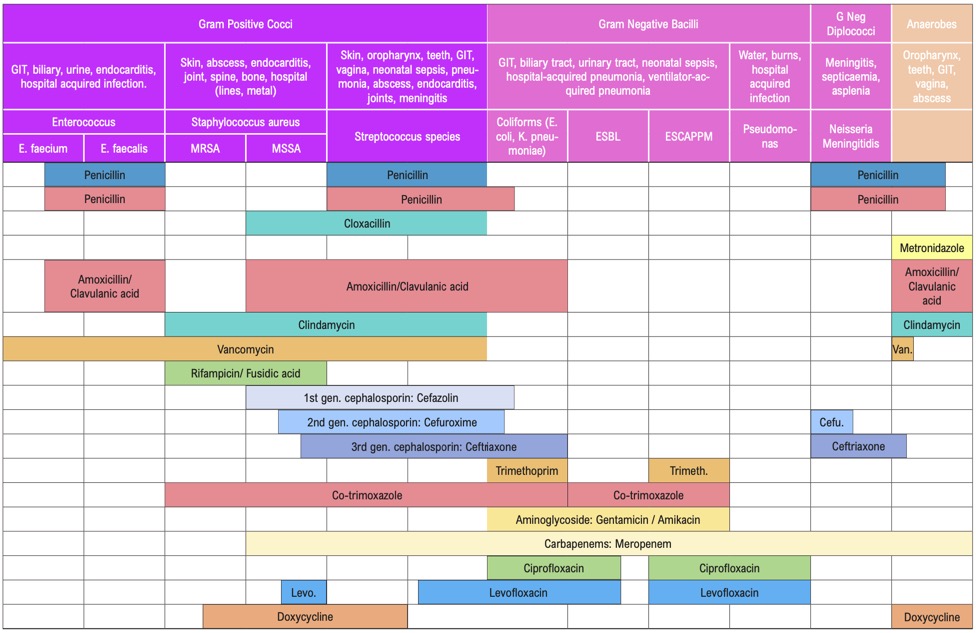
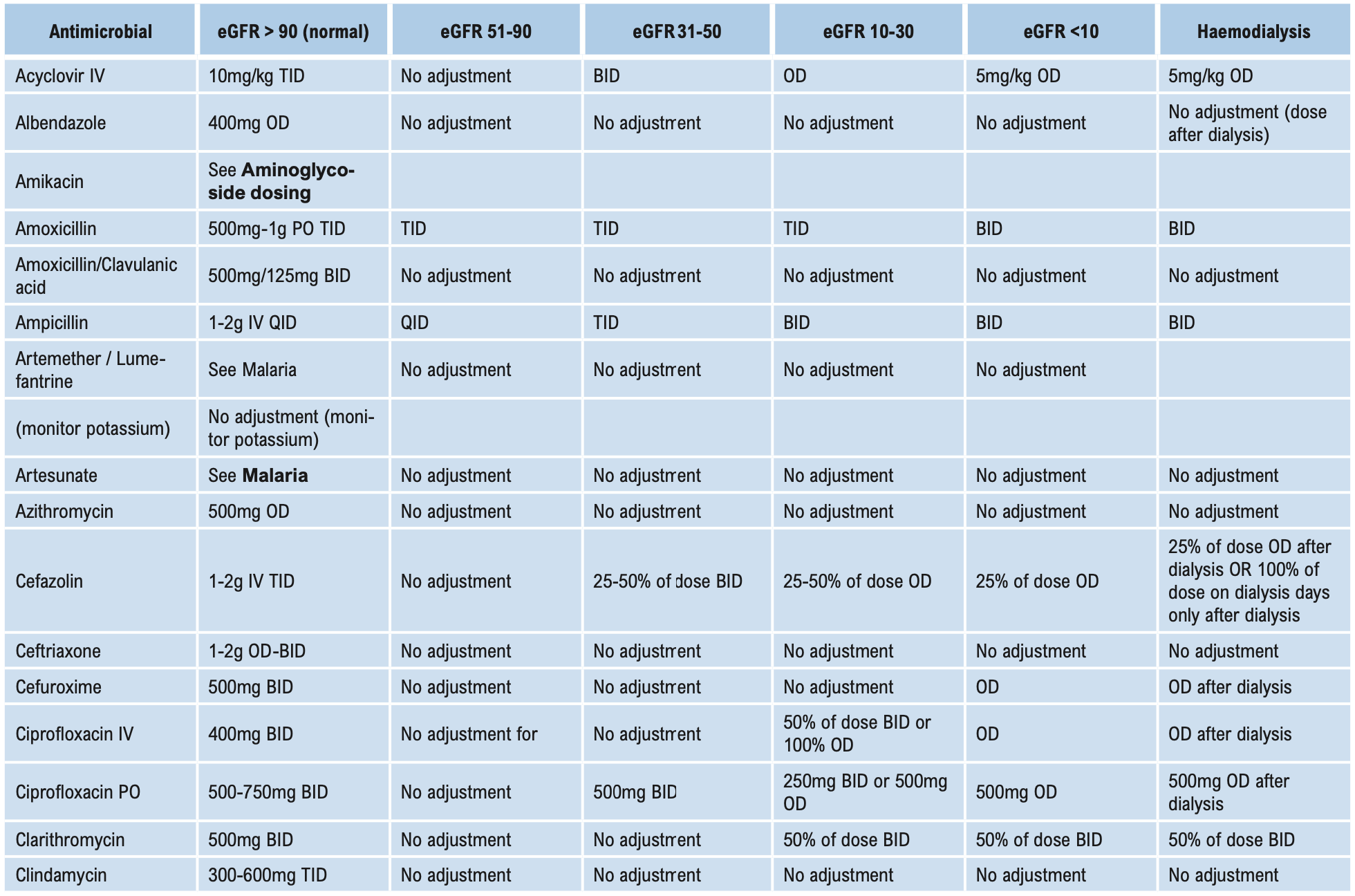
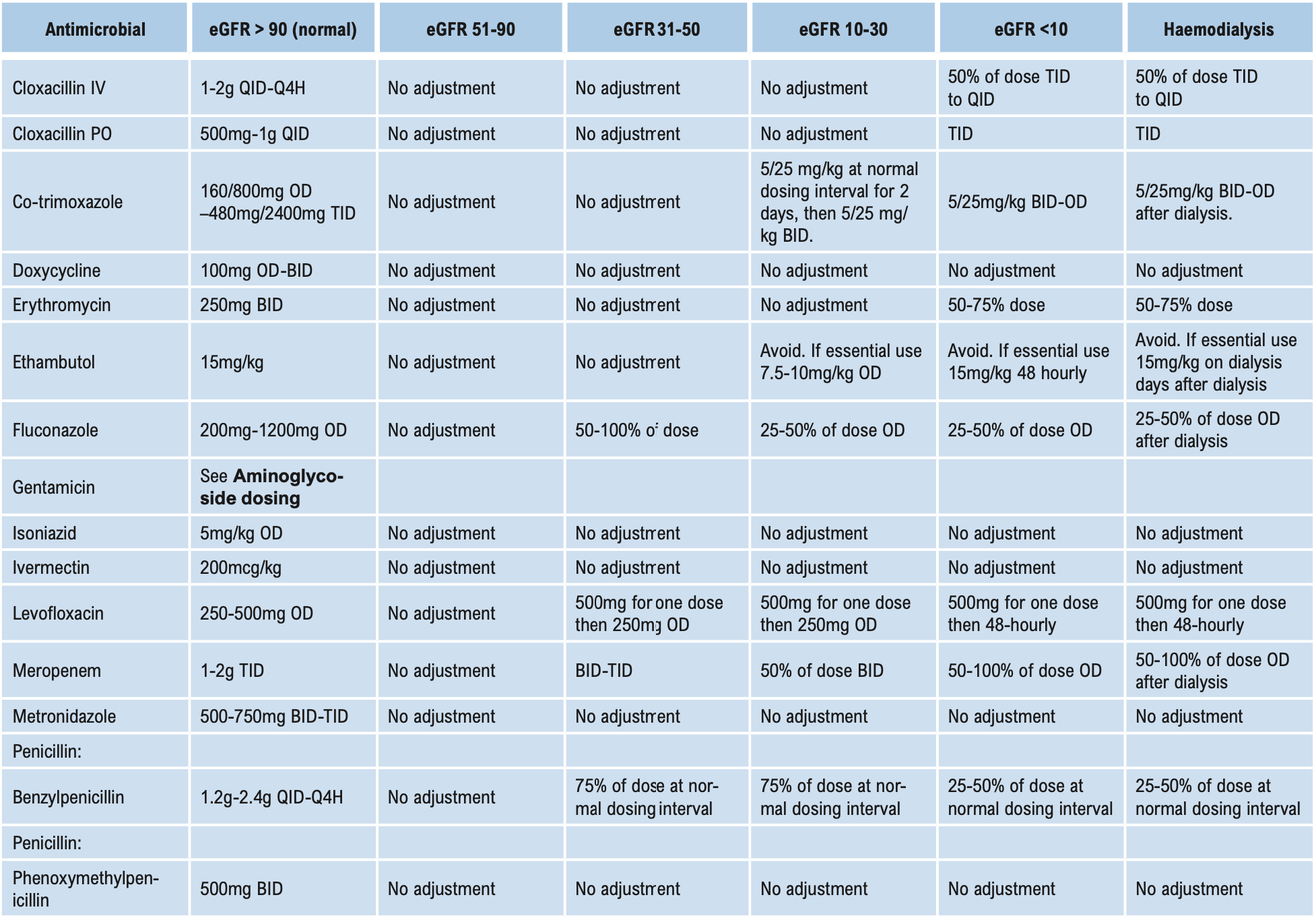
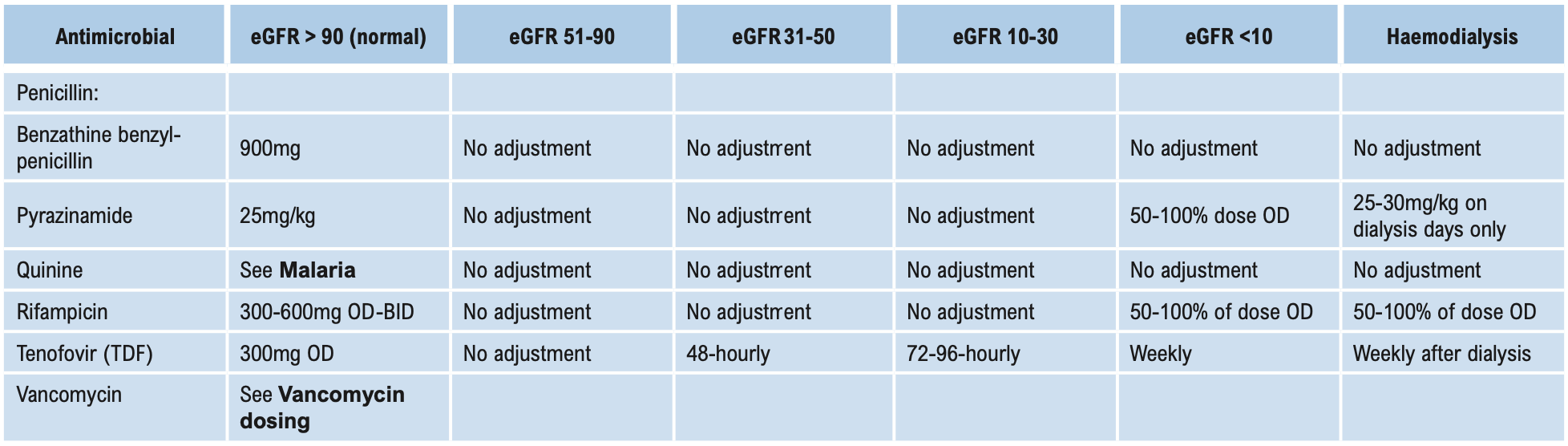
Β-LACTAMS
Penicillins, cephalosporins, monobactams and carbapenems have a β-lactam ring in their molecular structure. These bactericidal antibiotics act primarily on the bacterial cell wall. Although some bacteria produce β-lactamases and therefore have developed resistance, these drugs on the whole remain useful in treating many different types of infections.
PenicillinsPenicillin is active against Streptococci spp., Neisseria, Spirochaetes, some anaerobes including Clostridia and a few other organisms. Most Staphylococcus aureus isolates are intrinsically resistant to penicillin and there is growing resistance to cloxacillin. The prevalence of penicillinase-producing Neisseria gonorrhoeae is on the increase. There are reports of decreased susceptibility of pneumococci and streptococci to penicillin from other parts of the world. The only serious disadvantage of penicillins is the potential for hypersensitivity reaction.
A. Penicillins
-
Benzylpenicillin (also known as crystalline penicillin or penicillin G)
-
For intravenous (IV) use and needs to be given frequently (4-6 hourly).
-
-
Procaine Penicillin
-
Intramuscular (IM) preparation with a longer duration of action. Needs to be administered less frequently i.e. daily.
-
-
Benzathine Penicillin
-
Intramuscular preparation, providing low levels of penicillin in the circulation for 3-4 weeks.
-
-
Phenoxymethyl Penicillin (also known as penicillin V)
-
An oral preparation, intrinsically less active than Benzylpenicillin.
-
Penicillin is the drug of choice for the treatment of the following infections:
- Streptococcal infections e.g. tonsillopharyngitis
- Infections due to Streptococcus pneumoniae
- Meningococcal infections e.g. meningitis, septicaemia
- Syphilis
- Clostridial infections, Diphtheria
- Leptospirosis
B. Aminopenicillins
Ampicillin and amoxicillin are destroyed by staphylococcal β-lactamases but have a slightly broader spectrum than penicillins because of their activity against some Gram-negative bacilli such as E. coli, Salmonella spp. and Shigella spp. They also have better activity against H. influenzae and enterococci compared with penicillin.
Although previously sensitive, resistance to these drugs among E. coli is now widespread. Many strains of H. influenzae also produce β-lactamases, which can destroy these drugs.
Amoxicillin is better absorbed than ampicillin and has a longer half-life and hence is preferred for oral therapy. These drugs are used in the empirical treatment of respiratory infections and in the treatment of susceptible urinary tract infections. They may also be used for typhoid fever.
C. Anti–Staphylococcal Penicillins
These are narrow spectrum penicillins, resistant to Staphylococcal β-lactamases. Methicillin, oxacillin, and cloxacillins fall into this category. Of these, only cloxacillin, flucloxacillin and dicloxacillin are clinically useful and are to be used only for proven or suspected staphylococcal infections.
Cloxacillin, suitable for oral administration, can cause cholestatic jaundice in some patients.
Some Staphylococci have developed resistance to this group, by mechanisms other than β-lactamase. These methicillin-resistant Staphylococcus aureus (MRSA) will be resistant to all other β-lactams (i.e. all penicillin, ceph- alosporins, monobactams and carbapenems).
D. Anti–Pseudomonal Penicillins
These are newer penicillins with a high grade of activity against Gram-negative bacteria including pseudomonas, e.g. piperacillin, ticarcillin
E. β-lactam and β-lactamase inhibitor combinations
Amoxicillin can be combined with clavulanic acid, which itself has minimal antibacterial activity but inhibits β-lactamase effectively so that amoxicillin can still be used against β-lactamase-producing bacteria. Amoxicillin/clavulanic acid combination can cause cholestasis. Combinations utilising sulbactam are more expensive and so should be used only while treating infections with known β-lactamase producers.
Note: Hypersensitivity to any penicillin implies the potential for hypersensitivity to all penicillins. See Beta-lactam Allergy below.
Cephalosporins
The cephalosporins have been traditionally divided into “generations” based on their spectrum of activity. In general, cephalosporins are less prone to hypersensitivity reactions, are more stable to staphylococcal penicillinases and have a broader spectrum than penicillins. However, they have very little action against enterococci. None of the cephalosporins available in Timor-Leste have action against MRSA. Cephalosporins also have been shown to select out MRSA, vancomycin-resistant enterococci, and ceftriaxone-resistant Gram-negative bacilli. Therefore, indications for their use should be limited.
-
First generation cephalosporins include cephalexin (oral), cephalothin and cephazolin (parenteral). The spectrum of activity is similar for each, being effective against penicillinase-producing Staphylococci and other Gram-positive cocci (except MRSA and enterococci) and a few Gram-negative enteric bacilli. There is no special advantage for any one first generation cephalosporins over another. They are not usually the first choice for any infection, although are the first choice for most surgical prophylaxis. They may be used in some patients with penicillin hypersensitivity, see Beta-lactam Allergy below.
-
Second generation cephalosporins include (among others) cefuroxime and cefaclor (oral). These are more stable to some Gram-negative β-lactamases. Their activity against Gram-positive organisms is similar to, or less than, that of the first generation cephalosporins and they have varying degrees of activity against anaerobes. These drugs have a limited role in therapy and are more expensive than the first generation cephalosporins.
-
The major activity of the third generation cephalosporins (e.g. ceftriaxone, ceftazidime, cefotaxime) is against Gram-negative bacilli. They have some activity against Gram-positive cocci and their activity against anaerobes varies. A major advantage of these agents is their ability to reach the central nervous system. Ceftazidime has the additional benefit of specific anti-pseudomonal activity. Ceftriaxone and cefotaxime are useful in hospital-acquired and any other Gram-negative septicaemia and meningitis.
Monobactams (e.g. Aztreonam) and Carbapenems (e.g. Meropenem)
-
Aztreonam is active against Gram-negative bacteria including pseudomonas and β-lactamase-producing Enterobacteriaceae.
-
Carbapenems have a much broader spectrum, including Gram-positive, Gram-negative and some anaerobic bacteria. They are the agents of choice for ESBL (extended spectrum β-lactamase-producing organisms).
Aminoglycosides
This group of antibiotics (including gentamicin, tobramycin, amikacin, and streptomycin) act by inhibiting protein synthesis in bacteria. They have good activity against aerobic Gram-negative bacilli, including Brucella. When given together with penicillins, they have good activity against Enterococci. Streptomycin in combination is also useful against mycobacteria. Aminoglycosides are not absorbed when given orally and should be administered parenterally for systemic effects. Aminoglycosides are ototoxic and nephrotoxic. The therapeutic index is low and blood levels need to be monitored if used for either directed or empirical therapy for longer than 3 days (see Aminoglycoside dosing below). Despite this disadvantage, they are used widely for their action on Gram-negative bacilli. Gentamicin is the least expensive and is the aminoglycoside of choice for empirical treatment of Gram-negative infection including nosocomial infections. Due to high gentamicin resistance levels in Timor-Leste amikacin is the preferred aminoglycoside in septic shock.
The primary indication for aminoglycosides is as short-term empirical therapy pending the outcome of investigations. Their value as empirical drugs relates to their rapid bactericidal activity and the comparatively low levels of resistance in many community and healthcare-associated Gram-negative pathogens. When used empirically, no further doses should be given beyond 72 hours and if ongoing empirical IV therapy is required (i.e. an organism is not grown) therapy should be changed to an alternative, less toxic drug such as ceftriaxone.
Aminoglycosides are indicated for directed therapy in only a few circumstances. These include, but are not restricted to:
- Infections when resistance to other safer antimicrobials has been shown
- Low doses as synergistic treatment for streptococcal and enterococcal endocarditis.
Monitoring plasma concentrations of aminoglycosides is recommended in these patients and should commence on the first dose of directed therapy. See Aminoglycoside dosing below.
Fluoroquinolones
These antibiotics (e.g. ciprofloxacin, norfloxacin, levofloxacin) act by inhibiting DNA synthesis within bacteria. Their greatest activity is against aerobic Gram-negative bacilli including Pseudomonas spp., Haemophilus spp., and Gram-negative cocci such as Moraxella and Neisseria spp. Adverse effects include gastrointestinal side effects, hepatotoxicity, CNS toxicity, prolongation of the QT interval, tendonitis, and tendon rupture. Resistance occurs rapidly, so use should be restricted to where there is no alternative.
Gusmao dos Santos C, Francis J, Guterres J, Janson S, Lopes N, Marr I, et al. HNGV Antibiotic guidelines writing group. Antibiotic guidelines Hospital Nacional Guideo Valadares. Timor-Leste; 2016