Use of ARVS in Children
exp date isn't null, but text field is
More than 90% of HIV-infected children acquire their infection through mother to child transmission of HIV (vertical transmission). Thus, elimination of new HIV infections among children through effective PMTCT interventions should be prioritized. HIV disease progression occurs very rapidly in the first few months of life in infants acquiring HIV in utero, often leading to death. The importance of early infant diagnosis (EID) of HIV infection and early initiation of ART can therefore not be overemphasised.
Early Infant Diagnosis
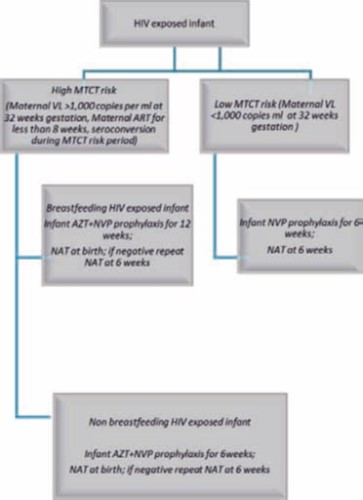
Birth PCR where available will be done within 48hrs of birth ONLY for high MTCT risk infants. ART initiation is recommended as soon as birth PCR results are available. For babies who test HIV positive at birth ALWAYS retest and confirm results with repeat PCR but retesting should not delay ART initiation. Babies who test negative at birth (birth PCR) or not tested MUST be tested at 6 weeks. Infants at high risk of transmission will receive dual ARVs (AZT and NVP) for 12 weeks as prophylaxis if breastfeeding and 6 weeks if not breastfeeding. Cotrimoxazole must be started from 6 weeks of age even in babies on longer duration of prophylaxis and continued through adolescence.
All infants with unknown or uncertain HIV exposure being attended to at health-care facilities at or around birth or at the first postnatal visit (usually 4-6 weeks) or other child health visit should have their HIV exposure status ascertained. This can be done in by:
- Asking if the mother knows her HIV status or is on ART
- Checking the hand held child health card for information on maternal HIV status
- Performing a rapid HIV test on the mother
- Performing a rapid HIV test on the baby - N.B. this can be used to assess HIV exposure only in infants less than 4 months of age. HIV exposure status in infants and children 4-18 months of age should be ascertained by undertaking HIV serological testing in the mother
Where virological testing is not available for children under 18 months of age, a presumptive diagnosis of severe HIV disease should be made if the infant is confirmed HIV antibody positive and:
- Diagnosis of any AIDS-defining condition(s) can be made, or
- The infant is symptomatic with two or more of the following:
- Oral thrush
- Severe pneumonia
- Severe sepsis
Infants under 18 months of age with clinically diagnosed presumptive severe HIV should be started on ART. Confirmation of HIV diagnosis should be obtained as soon as possible.
Recommendations for Antibody Testing in Infants
Antibody tests (rapid and laboratory-based ELISA) are the preferred method of diagnosis for HIV infection for children over 18 months of age.
In a child under 18 months who has never been breastfed and HIV antibody tests are negative, this child is uninfected and virological testing is indicated only if clinical signs or subsequent events suggest HIV infection.
In a child under 18 months who has not breastfed for more than six weeks, HIV antibody tests that are negative mean the child is uninfected.
HIV antibody tests that are positive at any age under 18 months identify those infants who need virological tests (i.e., the child is HIV exposed but needs definitive test with HIV DNA PCR to confirm HIV infection).
Care of an HIV Exposed Infant
Initial care
Care for HIV-exposed infants should include the following:
- Make sure HIV-exposed infants are entered into the "HIV exposed follow-up register".
- All HIV-exposed infants should have a HIV DNA PCR testing performed from six weeks of age or at the earliest possible time thereafter if 6 weeks testing is missed
- Cotrimoxazole prophylaxis should be given from six weeks of age until the HIV status of the infant is known. If HIV infection is confirmed, continue cotrimoxazole and commence on ART
- Monthly follow up visits are recommended, but more frequent visits may be needed if problems are detected
During these visits the following services should be provided:
- Growth monitoring and promotion
- Developmental assessment
Counselling on infant and young child feeding:
- Counselling and support for the HIV infected mother to adhere to ART is crucial
- Weaning should not be abrupt, but rather should be gradual over a one month period.
- HIV-infected infants diagnosed by virological testing or infants with symptoms suggestive of HIV should continue breastfeeding for as long as possible
- Immunisations should be given according to the national guidelines. The BCG vaccination should still be given at birth, but BCG should not be given to children with symptomatic HIV infection
- Always look for and treat opportunistic infections.
Management of an HIV Infected Child Using ARVs
Infants and young children have an exceptionally high risk of poor outcomes from HIV infection.
The goal of ART for children is to increase survival and decrease HIV related morbidity and mortality.
Criteria to initiate ART in children
Test earlier, test closer (using early infant diagnosis POC where available) and treat earlier. ART should be initiated in ALL children living with HIV, regardless of WHO clinical stage and CD4 count. Children under 5 years or with WHO clinical stage III/IV or CD4 < 25% (< 5 years) or ≤350 (>5 years) should be a priority.
Issues to consider in initiating ART in children
Psychosocial factors: It is important to identify and counsel at least one dedicated caregiver who can supervise and/or give medicines. Disclosure: The process of disclosure to the child should be initiated as early as possible, usually from as early as 5 - 7 years of age. Adherence is good in children who know their status and are supported to adhere to medicines.
Recommended first-line treatment for children
|
|
Preferred First line treatment |
Alternative first line treatment |
Special circumstances |
|
Neonates (up to 6 weeks) |
Zidovudine (AZT) + Lamivudine (3TC) + Raltegravir (Ral)* |
AZT + 3TC + NVP |
AZT+ 3TC + Lopinavir/ritonavir (LPV/r) |
|
Children> 6 weeks |
AZT+ 3TC + Lopinavir/ritonavir (LPV/r) |
AZT + 3TC + NVP ABC + 3TC + LPV/r ABC + 3TC + NVP |
|
|
Children and adolescents >25kg |
ABC+ 3TC + Dolutegravir (DTG)** |
ABC + 3TC + LPV/r |
ABC+ 3TC+ Efavirenz (EFV)*** AZT+ 3TC + Lopinavir/ritonavir (LPV/r)**** |
*For the shortest time possible (ideally for 2 weeks with transition to LPV/r syrup or granules). To allow for convenience and to align with the EPI schedule, RAL in neonates can be given for the first 6 weeks of life with substitution to LPV/r at 6 weeks of age until dosage formulations of DTG become available.
**For age and weight groups with DTG approved dosing and where LPV/r is not available
***From 3 years of age
****In cases where no other alternatives are available
Monitoring children on ART
- Check haemoglobin after at least 6-8 weeks if on zidovudine
- Use urine dipsticks to check for glycosuria and estimated glomerular filtration rate (eGFR) and/or serum creatinine when on tenofovir
- Check for alanine aminotransferase (ALT) when on nevirapine
- Check CD4 count every 6 months
- Conduct viral load testing once every year or when clinical signs are suggestive of treatment failure
Recommended Second Line Treatment in Children
Definition of treatment failure in children
Clinical Failure:
New or recurrent clinical event indicating advanced or severe immunodeficiency (WHO clinical stage 3 and 4, clinical condition with exception of TB) after 6 months of effective treatment
Immunological failure:
- In children younger than 5 years: CD4 levels persistently below 200 cells/mm3 or CD4 percentage <10%
- Children older than 5 years: CD4 levels persistently below 100cells/mm 3
Virological failure:
- Plasma viral load above 1000 copies/ml based on two consecutive viral load measurements after 3-months, with adherence support.
- OR if using dry blood spot technology, a viral load above 3000 copies/ml based on two consecutive viral load measurements after 3 months, with adherence support.
Recommended 2nd and 3rd Line ART regimens
|
Failing first line |
Second line |
Third line |
|
ABC + 3TC + LPV/r |
AZT +3TC +DTG |
DRV /r + 2NRT1s |
|
ABC + 3TC + DTG |
TDF +3TC +LPV/r |
DTG+DRV/r + 2NRT1s |
|
ABC + 3TC + EFV |
TDF + 3TC + DTG |
|
|
TDF + 3TC + DTG |
AZT+ 3TC + ATV/r or LPV/r |
- Where dosage guidelines and appropriate formulations are available, DTG is preferred as first line in children
- DTG can also be used in 1st, 2nd and 3rd line
- EFV should not be used in children less than 3 years of age
- DRV should not be used for children younger than 3 years of age.
- DRV should be used with ritonavir boosting in those above 3 years of age
Discuss the child with your mentor IF NOT SURE OF SECOND LINE TREATMENT
Starting ART in children using FDCs
Refer to dosing table. Keep the following factors in mind regarding dosing:
- Medicine doses must be adjusted as the child
- Dosing is by
- Overdosing up to 10% is acceptable
- Scored tablets may be divided into two equal halves
- Tablets may be crushed and mixed with a small amount food or water and administered immediately .

