Crytococcal meningitis
exp date isn't null, but text field is
ICD10 CODE: B45
Crytococcal meningitis is an opportunistic infection caused by a fungus Cryptococcus neoformans.
In Uganda, cryptococcal meningitis (CM) associated mortality is up to 39%. Patients with a CD4 cell count of <100 are at the highest risk, so early screening and management is critical.
Screening In ART-Naive Patients
- Screen routinely for Cryptococcal Meningitis with the cryptococcal antigen (CrAg) test (a bedside finger prick test):
- All ART naive individuals with CD4 <100 cells/µL
- Patients on ART with viral load (VL >1000 copies/ml) or clinical (stage 3 or 4 disease) failure
- If serum CrAg negative and no signs of meningitis: start ART immediately (or switch regimen)
- If CrAg positive and/or signs or symptoms of meningitis (headache, presence of seizures, altered consciousness, photophobia, neck stiffness, and a positive Kernigs’ sign)
- Perform lumbar puncture and test for CSF CrAg (culture if possible)
- If CSF CrAg positive, diagnose and treat for Cryptococcal Meningitis
- If CSF CrAg negative but blood CrAg positive, give pre emptive treatment for asymptomatic cryptococcal disease or non CNS cryptococcal disease
Cryptococcal screening algorithm

ART timing with CCM

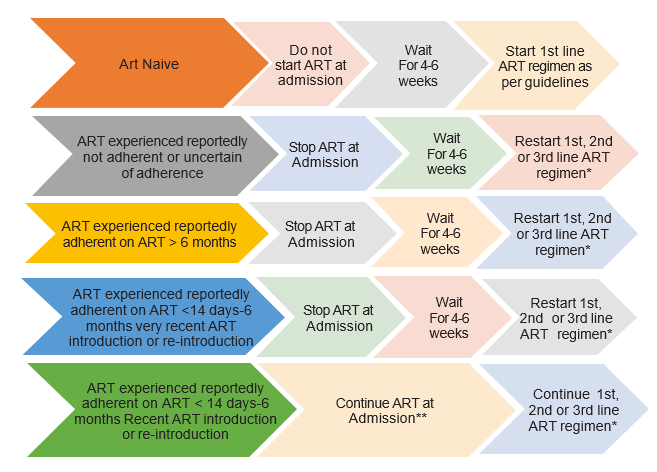
Decision on which ART regimen to restart should be made according to patient’s history, ART guidelines, HIV viral and genotypic resistance testing if possible. If it is considered likely that the patient has developed resistance to 1st line ARVs, then restart with 2nd line containing boosted PI or DTG is possible. ** Unless documented to have a suppressed viral load at time of admission or within the month prior to admission, in which case continue ART
Management of CCM
| Phase | Drug | Comments | LOC |
| Newly Diagnosed Patient | |||
| Induction Phase (2 weeks) |
Recommended: OR OR Fluconazole (1200 mg daily for adults, 12 mg/kg/day for children and OR |
Preventing Amphotericin toxicity: To prevent nephrotoxicity and hypokalemia, for patients on amphotericin deoxycholate do the following: • For electrolyte supplementation, two tablets daily of Magnesium Chloride 310 mg or slow Magnesium Chloride 535mg or Magnesium trisilicate 250mg while on amphotericin B |
HRRH H4 HRR |
| Consolidation phase (8 weeks) |
Fluconazole 800mg/day OR (6-12mg/kg/ day in children and adolescents) |
Initiate ART 4–6 weeks after starting CM treatment and there is clinical response to antifungal therapy. | H4 |
|
Maintenance Phase (18 months) |
Fluconazole 200mg/day OR (6 mg/kg/day up to 200mg in children and adolescents) |
Criteria to stop after a minimum of 18 months of maintenance phase: Adults: VL<1,000 copies/mm3 & CD4 200 or CD4 200 (if viral load not available) after 12 and 18 months Children: If CD4>25% or viral suppressed |
h4 |
| Relapse disease | |||
| Presents with a recurrence of symptoms of Meningitis and have a positive cerebrospinal fluid culture following a prior confirmed diagnosis of Cryptococcal Meningitis. | |||
| Evaluate for drug resistance: Send CSF to Central Public Health Laboratory (CPHL) for culture and sensitivity testing, if there are no drug resistance results, re-initiate the induction therapy for two weeks and complete other phases of treatment | |||
| Adequate control of elevated CSF pressure | |||
| • Control of increased intracranial pressure improves survival by 25% in persons with Cryptococcal Meningitis. • All patients with a CSF Pressure >250mm H2O will need a therapeutic LP the following day to reduce the CSF pressure to <200 mm. • In the absence of a manometer, one may use an IV giving set to create an improvised manometer measuring the height with a meter stick. Removing 20-30mL of CSF (even in the absence of a manometer) may be adequate to decrease CSF pressure. Most patients will need 2-3LPs during the induction phase. |
|||
