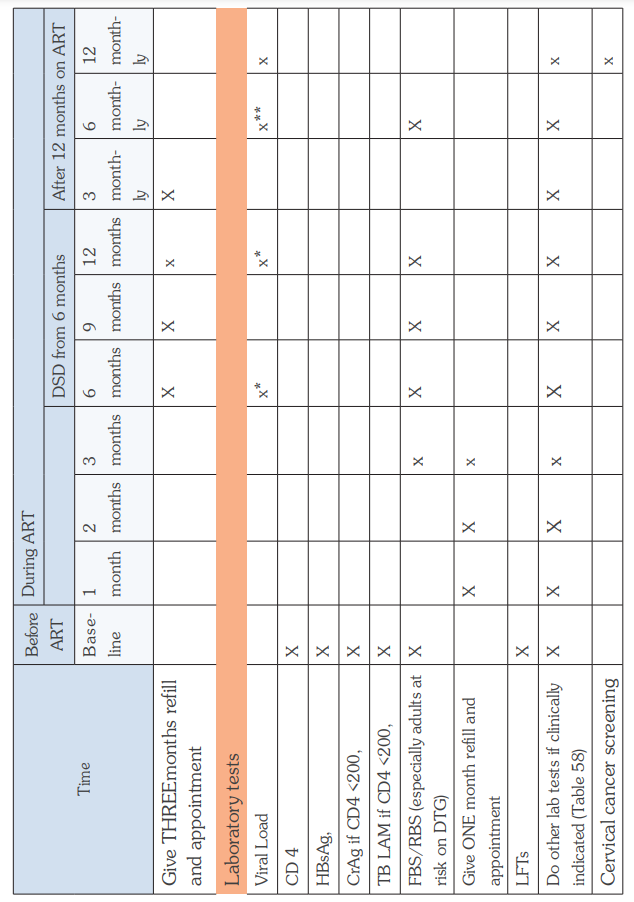
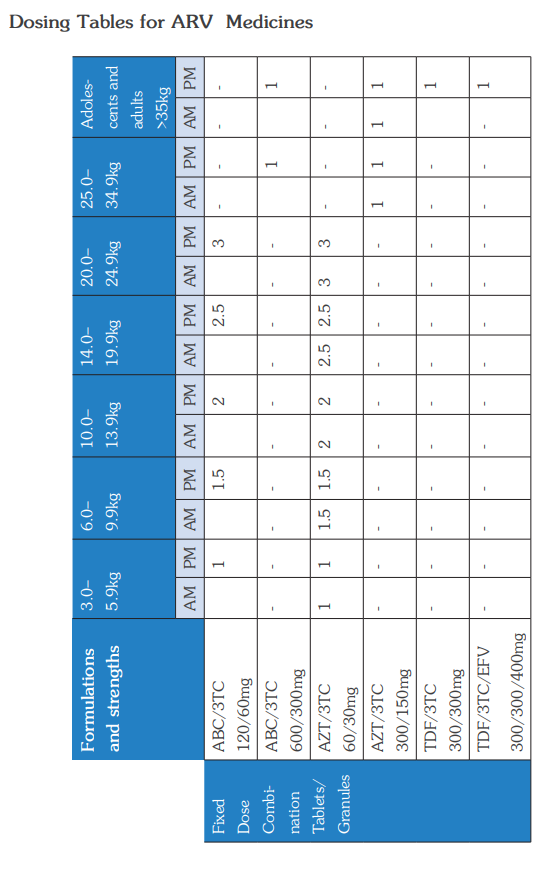
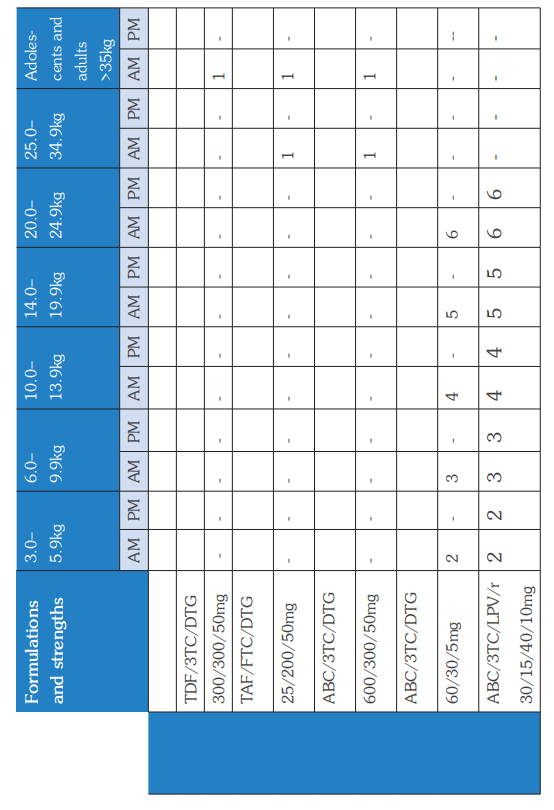
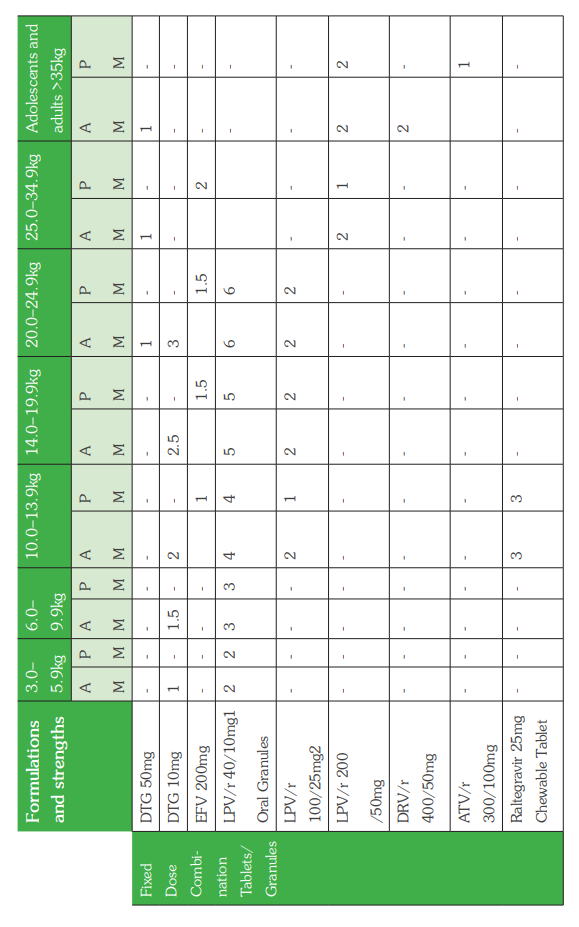
The WHO Clinical Staging of HIV for adults and children in the tables below shows the typical clinical features of HIV infection. The staging is based on demonstration of one or more opportunistic infections or key findings and correlates with disease progression and prognosis. Clinical staging should be performed at HIV diagnosis, on entry into HIV care, at ART initiation and at every visit hereafter to help guide patient care and monitor disease progress.
WHO Staging for HIV Infection and Disease in Adults and Adolescents
Clinical Stage I: Asymptomatic
- Asymptomatic
- Persistent generalised lymphadenopathy
Performance Scale 1: asymptomatic, normal activity
Clinical Stage II: Mild
- Moderate unexplained weight loss (< 10% of presumed or measured body weight)
- Minor mucocutaneous manifestations (seborrheic dermatitis, popular pruritic eruptions, fungal nail infections, recurrent oral ulcerations, angular cheilitis)
- Herpes zoster
- Recurrent upper respiratory tract infections (e.g. bacterial sinusitis, tonsillitis, otitis media, and pharyngitis)
And/or performance scale 2: symptomatic but normal activity
Clinical Stage III: Advanced
- Unexplained severe weight loss (more than 10% of presumed or measured body weight)
- Unexplained chronic diarrhoea for longer than 1 month
- Unexplained persistent fever (intermittent or constant for longer than 1 month)
- Persistent oral candidiasis
- Oral hairy leukoplakia
- Pulmonary tuberculosis
- Severe bacterial infections (such as pneumonia, pyomyositis, empyema, bone or joint infection, bacteraemia, meningitis)
- Acute necrotizing ulcerative stomatitis, gingivitis or periodontitis
- Unexplained anaemia (below 8 g/dl), neutropenia (below 0.5×109 per litre), or chronic thrombocytopenia (below 50× 109 per litre)
And/or performance scale 3: Bed ridden for less than 50% of the day during the last month
Clinical Stage IV: Severe
- HIV wasting syndrome
- Pneumocystis jirovecii pneumonia (PCP)
- Recurrent severe bacterial pneumonia (>2 episodes within 1 year)
- Toxoplasmosis of the brain
- Cryptosporidiosis with diarrhoea for longer than 1 month
- Chronic isosporiasis
- Extrapulmonary cryptococcosis including meningitis
- Cytomegalovirus infection (retinitis or infection of other organs other than liver, spleen or lymph nodes)
- Chronic oro-labial, genital or ano-rectal herpes simplex virus (HSV) infection of >1 month
- Progressive multifocal leukoencephalopathy (PML)
- Any disseminated endemic mycosis such as histoplasmosis, coccidioidomycosis
- Candidiasis of the oesophagus, trachea, bronchi, or lungs
- Disseminated non-tuberculous mycobacterial infection
- Recurrent septicaemia (including non-typhoid salmonella)
- Extrapulmonary tuberculosis
- Lymphoma (cerebral or B-cell non-Hodgkin)
- Invasive cancer of the cervix
- Kaposi sarcoma
- HIV encephalopathy
- Atypical disseminated leishmaniasis
- Symptomatic HIV-associated nephropathy or symptomatic HIV associated cardiomyopathy
And/or performance scale 4: Bed-ridden for more than 50% of the day during the last month
Differential diagnosis
- TB
- Untreated diabetes mellitus
- Malnutrition
- Cancer
- Other chronic diseases